Abstract
Introduction: Failure of hypomethylating agents (HMAs) in patients with myelodysplastic syndromes (MDS) is associated with dismal survival and there are no currently approved therapies in this setting. We recently presented the results of a multi-center phase 1b study of the immune checkpoint blocker (ICB) ipilimumab given as monotherapy after HMA failure in 29 MDS patients. Eight doses of 3mg/kg administered in induction and maintenance phases were safe and well tolerated. Very limited data has been reported on immunologic correlates of response after ICB therapy among patients with hematologic malignancies. Here we present a detailed analysis of the immunological correlates of achieving clinical benefit with ipilimumab.
Methods: We defined patients who achieved marrow complete response (mCR) or prolonged stable disease (PSD) for ≥46 weeks as having achieved meaningful clinical benefit (MCB) for analyses of immunologic studies. 11 patients were treated during the dose escalation phase (6 patients at 3mg/kg dose and 5 patients at 10 mg/kg), while another 18 patients were treated in the dose expansion part at the 3 mg/kg dose. Correlative immunologic studies were performed on mononuclear cells from both peripheral blood (PBMC) and bone marrow (BMMC) samples that were collected at baseline and at pre-determined timepoints after ipilimumab treatment (after induction cycle 2 and 4; after each maintenance cycle 1-4, and end of treatment or study discontinuation). Correlative assays included immunophenotyping of PBMCs and BMMCs by multicolor flow cytometry, as well as high throughput sequencing of the T cell antigen receptor (TCR) repertoire. T cell percentages, absolute numbers, and ratios were summarized by time point for patients and overall for healthy controls using descriptive statistics (mean, SD). Differences in log-transformed T cell values were compared between healthy controls and patients at pre-treatment using two-sample t tests. Differences from pre-treatment to cycle 2 and cycle 4 were estimated for patients and tested for positive or negative changes with paired t tests. Analyses were completed using R version 3.3.2.
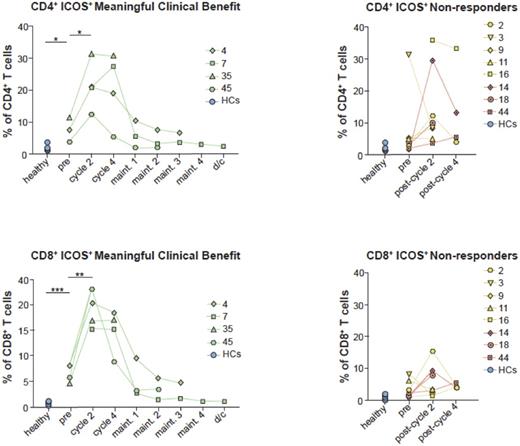
Results: MCB was observed in 28% of patients, 2 (7%) patients with mCR and 6 (21%) patients with PSD, respectively. Immunologic studies were performed on 16 patients who had samples available from at least two time points. Compared to solid tumor literature, we did not observe a correlation between early dynamic changes in serial absolute lymphocyte count and probability of achieving MCB. Expression of co-stimulatory molecules (ICOS - Inducible T Cell CO-Stimulator) and co-inhibitory molecules (PD-1 and CTLA-4) showed that a higher fraction of CD4+ and CD8+ T-cells in PB expressed ICOS in patients who achieved MCB following ipilimumab treatment (P=0.05 and P=0.01, respectively, Figure 1]. Similarly, expression of the immune co-inhibitory receptor CTLA-4 followed a similar pattern showing a trend of increased expression in both CD4+ and CD8+ T-cells (average increase of 6% and 2.4% respectively, P=ns.) over the first 4 cycles of ipilimumab treatment among patients who achieved MCB, followed by a gradual decrease. In contrast to CTLA-4, there was a wide range in frequencies of CD4+ and CD8+ T-cells expressing PD-1 in both patients and HC and PD-1 levels remained stable throughout ipilimumab treatment suggesting that the lack of T-cell activation in non-responders was not due to increased expression of other co-inhibitory molecules. There was a significantly higher frequency of CD3+CD4+Foxp3+ and activated effector Tregs (eTregs) prior to treatment which persisted throughout the examined time points and neither their levels, nor the CD8 T-cell to Treg (CD8/Treg) ratio were impacted by ipilimumab treatment.
Conclusions: These data represent, to our knowledge, the first detailed analysis of immunologic correlates of response to ICB therapy in MDS patients. Our data suggest that ICOS expression is positively correlated with achievement of MCB with single agent ipilimumab in patients with MDS and should be further explored as a biomarker for response. The results of the high throughput sequencing of the T cell antigen receptor (TCR) repertoire are currently being analyzed and will be presented at the meeting.
Smith: Celgene: Other: DSMB member; Jazz: Membership on an entity's Board of Directors or advisory committees. Zeidner: Tolero Pharmaceuticals: Honoraria; Agios: Honoraria; Tolero Pharmaceuticals: Research Funding; Takeda: Research Funding; Merck: Research Funding; Celgene: Honoraria. Frattini: Astellas: Honoraria; Lin Bioscience: Consultancy. Levy: Actinium Pharmaceuticals: Equity Ownership; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; Takeda: Consultancy, Speakers Bureau. Zeidan: AbbVie, Otsuka, Pfizer, Gilead, Celgene, Ariad, Incyte: Consultancy, Honoraria; Takeda: Speakers Bureau; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.